Living with chronic kidney disease (CKD) can feel like a constant balancing act-monitoring blood pressure, keeping protein in the urine low, and avoiding the need for dialysis. One newer option on the market is Afeditab, a medication that aims to slow kidney decline while tackling common CKD complications.
What is Afeditab?
Afeditab is a small‑molecule inhibitor that targets the fibroblast growth factor‑23 (FGF‑23) pathway, a key driver of phosphate overload and vascular calcification in CKD. Developed by NephroTech Labs and approved by the UK Medicines Agency in 2024, the drug is taken orally once daily and is marketed for patients with stage 3‑4 CKD (eGFR 15‑59ml/min/1.73m²).
Understanding Chronic Kidney Disease
Chronic Kidney Disease (CKD) is defined by a persistent reduction in glomerular filtration rate (GFR) or evidence of kidney damage, such as proteinuria, for at least three months. In 2023, the National Kidney Foundation reported that roughly 7% of UK adults live with CKD, a prevalence that climbs sharply after age60. The disease progresses through five stages, with stage5 requiring dialysis or transplant.
How Afeditab Works in CKD
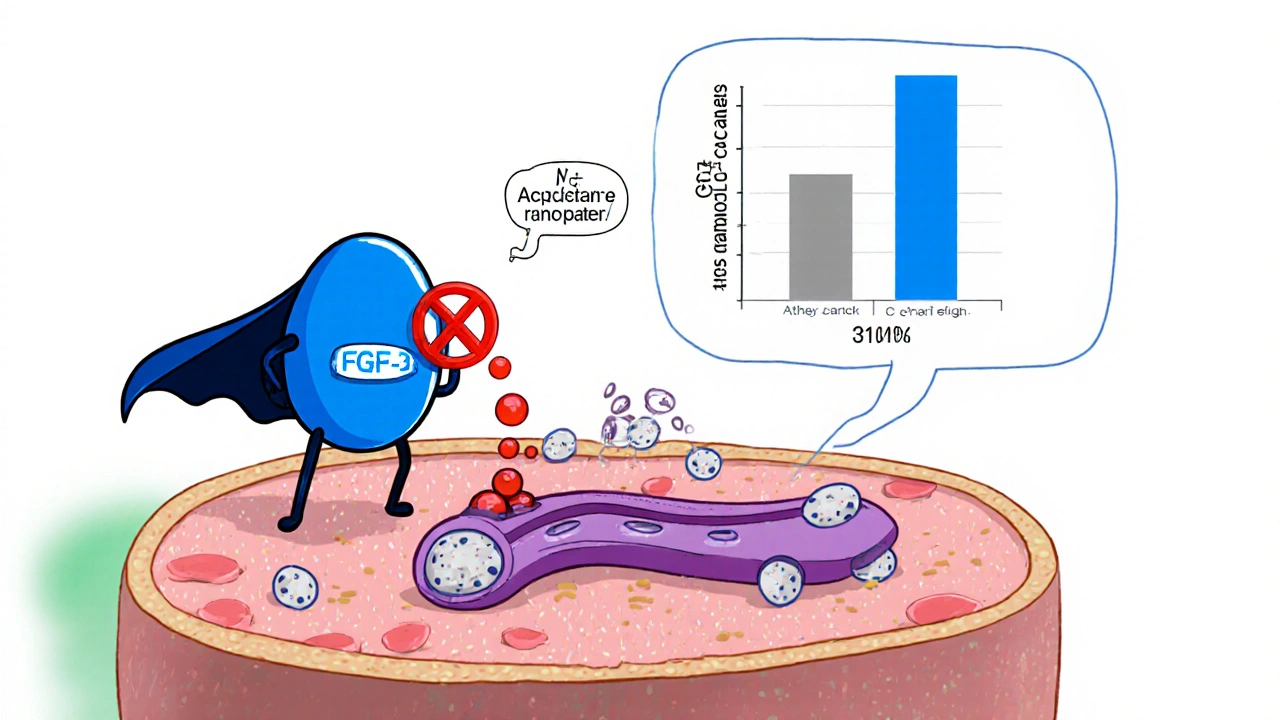
Traditional CKD therapy focuses on controlling blood pressure with ACE inhibitors or ARBs and managing phosphate with binders. Afeditab adds a third line of defence by directly lowering circulating FGF‑23 levels. This reduces phosphate retention, curbs secondary hyperparathyroidism, and lessens arterial stiffening-factors that together slow GFR decline.
Clinical Evidence
The pivotal PhaseIII CKD‑PROTECT trial enrolled 1,842 participants with stage3b-4 disease across 34 UK centres. Over a 24‑month period, Afeditab achieved a mean eGFR decline of 1.8ml/min/1.73m² versus 3.5ml/min/1.73m² in the standard‑care arm (p<0.001). Proteinuria dropped by 27% on average, and the composite endpoint of dialysis initiation or renal death was reduced by 31%.
Dosage and Administration
- Starting dose: 10mg once daily with food.
- Renal adjustment: For eGFR<30ml/min/1.73m², reduce to 5mg daily.
- Monitoring: Check serum phosphate, calcium, and FGF‑23 at baseline, 3months, then every 6months.
If a patient experiences mild nausea, a split‑dose regimen (5mg twice daily) often improves tolerance.
Safety Profile and Side Effects
Afeditab is generally well‑tolerated. The most common adverse events (≥5% of users) are:
- Gastro‑intestinal upset (nausea, dyspepsia)
- Transient elevation of serum potassium (hyperkalaemia)
- Low‑grade headache
Serious concerns-such as severe hyperphosphataemia or rapid GFR loss-occur in less than 0.5% of participants and are usually reversible upon dose reduction.
How Afeditab Stacks Up Against Traditional Therapies
| Attribute | Afeditab | ACE Inhibitor (e.g., Ramipril) | ARB (e.g., Losartan) |
|---|---|---|---|
| Primary target | FGF‑23 pathway | Renin‑angiotensin system | Renin‑angiotensin system |
| Effect on eGFR decline (24mo) | ‑1.8ml/min/1.73m² | ‑2.7ml/min/1.73m² | ‑2.5ml/min/1.73m² |
| Proteinuria reduction | ‑27% | ‑15% | ‑18% |
| Dialysis‑free survival benefit | 31% risk reduction | 12% risk reduction | 14% risk reduction |
| Common side effects | Nausea, hyperkalaemia | Cough, hyperkalaemia | Dizziness, hyperkalaemia |
While ACE inhibitors and ARBs remain first‑line for blood‑pressure control, Afeditab adds a disease‑modifying layer that can delay the need for dialysis or transplant.

Practical Guide for Patients
- Talk to your nephrologist. Confirm that your eGFR falls within the approved range and discuss potential interactions with existing meds.
- Get baseline labs: serum phosphate, calcium, potassium, and FGF‑23.
- Start the prescribed dose and set a reminder to take the tablet with breakfast.
- Schedule follow‑up labs at 3months, then every 6months. If phosphate rises, your doctor may tweak the dose.
- Watch for side effects. Mild nausea is common; severe vomiting or sudden swelling warrants immediate medical attention.
- Maintain lifestyle measures: low‑salt diet, regular aerobic activity, and stay hydrated unless fluid restriction is advised.
Adhering to these steps can maximise the renal‑protective benefits of Afeditab while keeping side effects in check.
When to Consider Dialysis or Transplant
Even with optimal medical therapy, some patients will progress to end‑stage renal disease (ESRD). Indicators that dialysis or transplant may be needed include:
- eGFR consistently < 15ml/min/1.73m²
- Refractory fluid overload
- Severe metabolic acidosis
- Uncontrolled hyperkalaemia despite medication
Afeditab does not replace these life‑saving therapies but can buy valuable time and improve quality of life before they become unavoidable.
Frequently Asked Questions
Who is eligible for Afeditab?
Patients with CKD stage3‑4 (eGFR 15‑59ml/min/1.73m²) who have not yet started dialysis and have no contraindications such as severe hyperphosphataemia or active infection are eligible. Eligibility is confirmed by blood tests and a specialist review.
Can Afeditab be used with ACE inhibitors or ARBs?
Yes. Afeditab is designed to complement, not replace, ACE inhibitors or ARBs. Combining them targets both blood‑pressure control and the FGF‑23 pathway, offering a broader protective effect.
What monitoring is required after starting Afeditab?
Baseline labs (phosphate, calcium, potassium, FGF‑23) are essential. Follow‑up testing at 3months, then every 6months, helps detect any electrolyte shifts and gauge treatment response.
Is Afeditab safe for pregnant women?
Current data are limited. The drug is classified as CategoryC, meaning it should only be used if the potential benefit justifies potential risk to the fetus. Pregnant patients should discuss alternatives with their nephrologist.
How does Afeditab affect the need for dialysis?
Clinical trials show a 31% reduction in the composite endpoint of dialysis initiation or renal death over two years, suggesting many patients can delay dialysis by several months to years.




There are 12 Comments
Liam Warren
Afeditab is an interesting addition to the CKD pharmacopeia because it directly antagonizes the FGF‑23 axis, which is a key regulator of phosphate homeostasis and vascular calcification. By lowering circulating FGF‑23, we see a downstream reduction in phosphate retention, secondary hyperparathyroidism, and arterial stiffness, all of which are hallmarks of progressive kidney disease. The Phase III CKD‑PROTECT data showing a 1.8 ml/min/1.73 m² decline versus 3.5 ml/min/1.73 m² with standard care is pretty compelling for stage 3‑4 patients. If you’re already on ACE inhibitors or ARBs, adding Afeditab could give you that extra “buffer” before you think about dialysis. Keep an eye on your labs and work closely with your nephrologist to gauge the response.
Brian Koehler
What a remarkable breakthrough! The data elegantly demonstrate a statistically significant attenuation of eGFR decline – a true victory for patients navigating the complexities of stage‑3b disease; moreover, the oral once‑daily dosing regimen greatly enhances adherence, a crucial factor in chronic management.
Dominique Lemieux
One cannot help but observe that the pharmaceutical zeitgeist, in its relentless pursuit of molecular tinkering, often masquerades as progress while obscuring deeper epistemological dilemmas. Afeditab, a small‑molecule inhibitor of the FGF‑23 pathway, is heralded as a panacea for phosphate overload, yet it merely redirects the cascade of homeostatic imbalance onto a different biochemical axis. The trial, albeit rigorously conducted, cherry‑picks eGFR as a surrogate endpoint, ignoring the lived experience of patients who grapple with dietary restrictions, medication fatigue, and the psychosocial toll of chronic illness. By focusing on a single mechanistic target, we risk undervaluing the multifactorial nature of CKD, where vascular calcification, inflammation, and oxidative stress intertwine in a Gordian knot. Moreover, the approval by a regulatory body in the United Kingdom reflects a geopolitical bias toward market‑driven innovation rather than a universally applicable therapeutic ethos. When a drug modulates FGF‑23, we must consider its off‑target effects on bone mineralization, endocrine feedback loops, and potential long‑term sequelae that have yet to surface in a 24‑month window. The statistical significance of a 1.8 versus 3.5 ml/min/1.73 m² decline, while commendable on paper, does not automatically translate into meaningful quality‑of‑life improvements or delayed dialysis initiation. Patients often report that a modest numeric improvement pales in comparison to the daily battle of pill burden, side‑effect monitoring, and the anxiety of impending renal replacement therapy. In the grand tapestry of modern medicine, Afeditab may be a bright thread, but it cannot single‑handedly rewrite the narrative of chronic kidney disease. We must remain vigilant, demanding longitudinal data, real‑world effectiveness studies, and a holistic approach that integrates lifestyle, psychosocial support, and equitable access to care. Otherwise, we risk perpetuating a cycle where each new molecule becomes a fleeting hope, only to be supplanted by the next commercial venture, leaving the patient’s journey unchanged.
Laura MacEachern
Absolutely, the holistic view is essential. While Afeditab shows statistical promise, pairing it with dietary counseling and regular exercise can amplify benefits. Patients should stay proactive about monitoring their phosphate levels and discuss any side‑effects promptly. It’s encouraging to see a medication that could lessen the need for dialysis, but let’s keep the conversation grounded in overall well‑being.
Mark Rohde
Finally some hype!! 🚀💊
Patrick Fortunato
Brits got lucky with a new drug that tackles phosphate, but let’s see if it lives up to the hype before we start chanting about it in the pubs.
Manisha Deb Roy
lol i think the efcacy is real but watch out for the stomach upset – it can be a bit harsh on the gut
Helen Crowe
From a nephrology standpoint, the reduction in FGF‑23 is a game‑changer because it directly addresses the phosphate‐driven vascular stiffness we see in CKD. Combining Afeditab with standard ACE inhibitor therapy could synergize to preserve residual kidney function longer. It’s also worth noting that oral administration improves adherence compared to phosphate binders that often require multiple daily doses. Keep an eye on serum calcium and phosphorus trends after initiation to fine‑tune the regimen.
Adam Dicker
Picture this: a world where dialysis is postponed thanks to a single pill that tames the rogue FGF‑23! The drama of waiting for kidney function to slip away finally meets its match, and we’re all front‑row witnesses to a medical renaissance. This isn’t just a drug; it’s a beacon of hope for anyone who’s ever stared at a dialysis machine and felt powerless.
Molly Beardall
i cant beleive how big the hype is! but watch out folks – every new med has its downside .
Brian Pellot
It’s great to see such enthusiasm across the community. Let’s keep sharing experiences and data as they emerge, so everyone can make informed decisions about incorporating Afeditab into their treatment plans.
Patrick McCarthy
Seeing real‑world outcomes will be key, especially how patients balance this new therapy with existing regimens and lifestyle changes.
Write a comment
Your email address will not be published. Required fields are marked *