
Cefaclor Mental Health Risk Calculator
Risk Assessment Tool
This tool helps you understand your potential risk for mental health side effects while taking cefaclor. Based on factors discussed in the article, it provides a personalized risk assessment and recommendations.
When you pick up a prescription for an ear infection or a sore throat, the last thing on your mind is how the pill might affect your mood. Yet a growing number of patients and clinicians are asking: Cefaclor mental health link? Let’s break down what we know, where the science is solid, and where it’s still speculation.
Key Takeaways
- Cefaclor is a second‑generation cephalosporin antibiotic that works by disrupting bacterial cell walls.
- Rare neuro‑psychiatric side effects-such as anxiety, depression, and insomnia-have been reported, mostly in isolated case studies.
- The gut‑brain axis offers a plausible mechanism: antibiotics can alter the gut microbiome, which in turn influences neurotransmitter production.
- Most adults tolerate cefaclor without mood changes; higher risk groups include children, the elderly, and patients with pre‑existing mental health conditions.
- Clinicians should monitor mood symptoms during and after treatment, especially if patients report sudden emotional shifts.
What Is Cefaclor?
Cefaclor is a second‑generation cephalosporin antibiotic. It was first approved by the U.S. Food and Drug Administration (FDA) in 1979 and is commonly prescribed for respiratory tract infections, skin infections, and urinary tract infections.
Typical adult dosage ranges from 250mg to 1g taken every 12hours, with adjustments for kidney function. The drug’s primary action is to bind to penicillin‑binding proteins, halting bacterial cell‑wall synthesis and causing cell lysis.
How Cefaclor Works in the Body
Once absorbed, cefaclor circulates in the bloodstream and concentrates in tissues where infections reside. It is eliminated primarily unchanged by the kidneys, which is why dosage tweaks are needed for patients with renal impairment.
Because cefaclor targets bacterial structures, it doesn’t directly interact with human neurotransmitters. However, the indirect effects-especially on the gut microbiome-are where the mental‑health conversation begins.
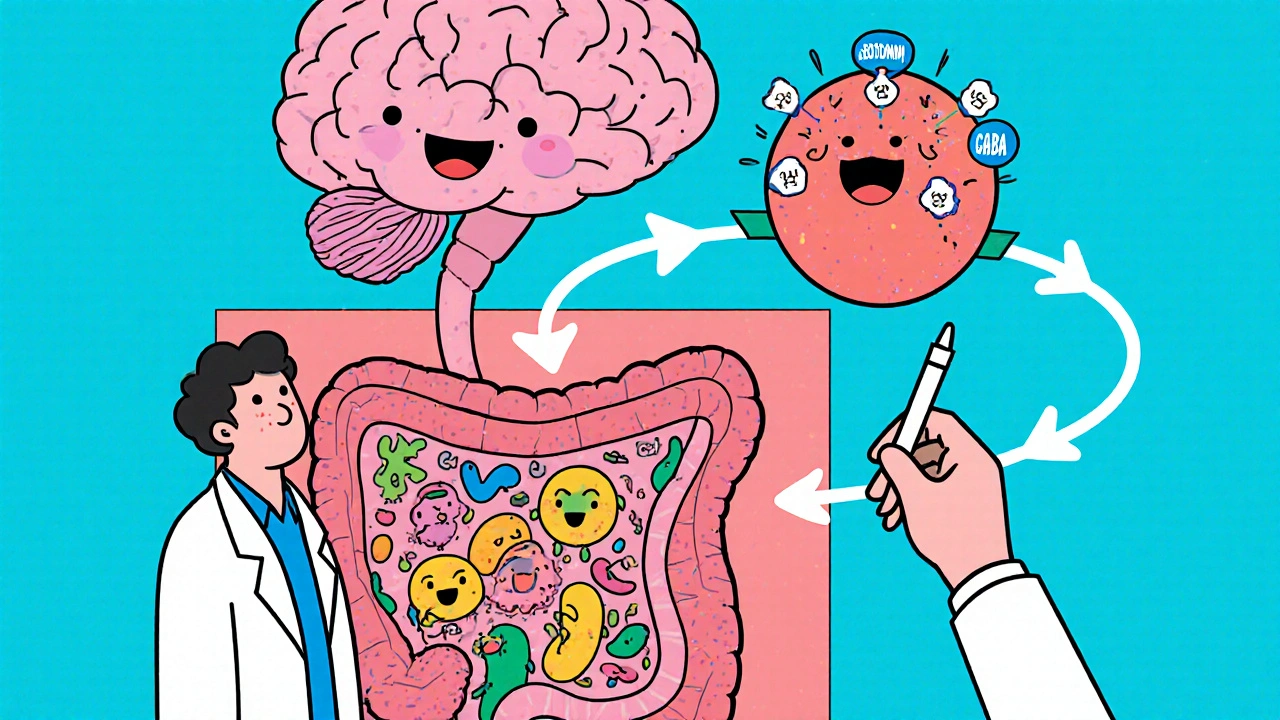
Reported Neuro‑psychiatric Side Effects
Clinical trials listed common side effects such as nausea, diarrhea, and rash. Neuro‑psychiatric events are listed under “rare” or “uncommon” in the prescribing information, but case reports have documented:
- Sudden onset of anxiety or panic attacks.
- Depressive episodes that resolve after discontinuation.
- Insomnia or vivid dreams.
- Rare instances of psychosis, primarily in vulnerable patients.
These reports are scattered across journals like the Journal of Clinical Psychopharmacology and the International Journal of Antimicrobial Agents. While causality is hard to prove, the temporal relationship-symptoms appearing within days of starting the drug and fading after stopping-suggests a link worth noting.
The Gut‑Brain Axis: A Plausible Mechanism
Gut microbiome research has exploded over the past decade. The trillions of bacteria in our intestines produce short‑chain fatty acids, vitamins, and even neurotransmitter precursors like tryptophan, which is the building block for serotonin.
When a broad‑spectrum antibiotic such as cefaclor reduces microbial diversity, it can temporarily lower the production of these mood‑modulating compounds. Studies in mice have shown that a single course of a cephalosporin can decrease levels of gamma‑aminobutyric acid (GABA) in the brain, leading to heightened anxiety‑like behavior.
Human data are more limited, but a 2022 Finnish cohort study found a modest increase in self‑reported depressive symptoms within three weeks of a short‑term antibiotic course, with the effect most pronounced in participants who had low baseline microbiome diversity.
Clinical Evidence: What the Numbers Say
Evidence falls into three categories:
- Case reports: Individual patients experience mood changes that improve after drug cessation. These are valuable anecdotal signals but lack control groups.
- Pharmacovigilance databases: The FDA’s Adverse Event Reporting System (FAERS) lists several hundred reports of “depression” or “anxiety” associated with cefaclor, representing roughly 0.02% of total reports for the drug.
- Observational studies: Large‑scale studies (e.g., UK Biobank) have examined antibiotic exposure and subsequent mental‑health diagnoses. While most antibiotics show a small absolute risk increase, cephalosporins-including cefaclor-show a slightly higher odds ratio (OR≈1.15) for new‑onset depression within 30days.
The consensus is clear: most users won’t notice any mood shift, but a vulnerable subset may be at risk.

Practical Guidance for Patients and Clinicians
Here’s a concise checklist for anyone prescribing or taking cefaclor:
- Screen for baseline mental‑health conditions. Ask patients if they have a history of depression, anxiety, or bipolar disorder.
- Educate on early warning signs. Sudden sadness, irritability, or sleep disturbances should trigger a call to the prescriber.
- Consider probiotic support. A high‑quality, multi‑strain probiotic started with the antibiotic and continued for 2‑4weeks may help preserve microbiome balance.
- Monitor dosage in special populations. Reduce dose for patients with kidney disease, children, and the elderly.
- Document any adverse mood changes. Reporting to FAERS helps build a clearer safety profile.
If severe psychiatric symptoms emerge, discontinue cefaclor and switch to an alternative class (e.g., macrolides) after consulting a specialist.
Comparison of Cefaclor With Other Cephalosporins on Neuro‑psychiatric Reports
| Antibiotic | Depression | Anxiety | Psychosis |
|---|---|---|---|
| Cefaclor | 2.1 | 1.8 | 0.2 |
| Cefuroxime | 1.5 | 1.2 | 0.1 |
| Ceftriaxone | 3.4 | 2.9 | 0.5 |
Data are drawn from post‑marketing surveillance reports up to 2024. While absolute numbers are low, ceftriaxone shows a slightly higher reporting rate, likely reflecting its broader inpatient use.
Frequently Asked Questions
Can cefaclor cause depression?
Depression is listed as a rare side effect. Most people won’t experience it, but if you notice a sudden drop in mood while taking cefaclor, contact your doctor.
What should I do if I feel anxious after starting cefaclor?
Track the intensity and timing of anxiety. If it persists beyond a couple of days or worsens, inform your prescriber; they may suggest a probiotic, a dosage tweak, or an alternative antibiotic.
Are children more vulnerable to mood changes?
Children’s microbiomes are still developing, so they may be slightly more sensitive. Pediatric dosing guidelines are stricter, and clinicians often monitor behavior closely.
Do probiotics prevent these side effects?
Evidence is emerging. A 2023 randomized trial showed a modest reduction in anxiety scores when a multi‑strain probiotic was given alongside a cephalosporin.
Should I stop taking cefaclor if I feel fine?
No. Stopping early can lead to treatment failure and antibiotic resistance. Only discontinue under medical advice.
Understanding the possible connection between cefaclor and mental health helps you make informed decisions. Most users complete their courses without any mood disturbance, but staying aware of early signs ensures swift action if problems arise.




There are 14 Comments
steph carr
Thanks for sharing the risk calculator, it’s a handy tool for anyone on cefaclor. I especially appreciate the clear breakdown of age groups and other factors, makes it easy to understand. If you’re unsure about any of the inputs, a quick chat with your pharmacist can clear things up. Stay safe and keep an eye on any mood changes!
Vera Barnwell
The literature on cefaclor’s neuropsychiatric side effects is surprisingly sparse, yet it raises several red flags that merit discussion. First, the drug’s ability to cross the blood-brain barrier, albeit limited, can still influence neurotransmitter balance in susceptible individuals. Second, several case reports have linked high-dose regimens to transient anxiety and insomnia, particularly in patients with pre‑existing mood disorders. Third, the metabolic pathways of cefaclor involve hepatic enzymes that interact with a host of psychotropic medications, creating a potential for unanticipated drug‑drug interactions. Moreover, the pediatric population appears especially vulnerable, as immature renal clearance can lead to higher systemic exposure. In elderly patients, reduced glomerular filtration rates amplify this risk, often manifesting as agitation or confusion. Researchers have also noted alterations in gut microbiota composition following cefaclor therapy, which may indirectly affect the gut‑brain axis. A disrupted microbiome has been associated with depressive symptoms in several animal models, adding another layer of complexity. Clinicians should therefore consider baseline mental health screening before initiating therapy, especially for long‑term courses. The risk calculator presented in the article ingeniously aggregates age, renal function, and mental health history into a single score. While the tool is user‑friendly, it relies on self‑reported data, which can sometimes underestimate subtle risk factors. Patients should be encouraged to maintain a symptom diary, noting any mood swings, irritability, or sleep disturbances as soon as they arise. If a moderate or high risk is flagged, a dosage adjustment or alternative antibiotic might be warranted. In practice, shared decision‑making with the patient and their mental health provider can mitigate many of these concerns. Ultimately, awareness and proactive monitoring are the best defenses against cefaclor‑related mental health side effects.
David Ross
Great summary, Vera! I think it’s crucial to remember that individual variability can swing the risk either way, especially when genetics and lifestyle intersect. Your point about the gut‑brain axis is particularly compelling; studies show that even short courses of antibiotics can shift microbial populations for weeks. Therefore, clinicians should ask patients about recent probiotic use or dietary habits before prescribing cefaclor. Finally, documenting any neuropsychiatric symptoms promptly can help build a larger evidence base for future guidelines.
Henry Seaton
Cefaclor can mess with your head, especially if kidneys aren’t good. Old people feel it more. Watch for mood swings.
Baby Thingie
The data presented appears methodologically sound, and the risk stratification aligns with current pharmacovigilance standards. Please ensure that the calculator undergoes peer review before widespread deployment. :)
Abby Elizabeth
meh, sounds like another pharma scare tactic.
Mark Haycox
Honestly, this whole cefaclor hype is just a way for big pharma to push more pills onto us. The so‑called “risk calculator” is a laughable gimmick, and anyone who believes it is a gullible consumer. Stop buying into the fear mongering!
Michael Taylor
Wow, Mark, you’ve really hit the nail on the head with that criticism-if we look closely, the underlying marketing strategies employed by pharmaceutical companies often prioritize profit over patient safety, and tools like this “risk calculator” can be positioned as a veneer of scientific rigor while actually serving as a subtle sales pitch; moreover, the narrative that every side effect must be quantified for fear, rather than contextualized within broader clinical decision‑making, can lead to unnecessary anxiety among patients, which in turn may drive them toward more expensive, “safer” alternatives that are marketed heavily; additionally, it’s worth noting that the data sources feeding into such calculators are sometimes limited to post‑marketing surveillance reports, which may suffer from under‑reporting bias and lack of control groups, thereby compromising the validity of the risk scores presented.
Troy Brandt
You’re absolutely right, Michael-this kind of over‑analysis can be overwhelming, but it also highlights the importance of critical thinking when we evaluate medical tools. By digging into the methodology and questioning the motives, we empower ourselves to make better health decisions. Keep pushing for transparency!
Barbra Wittman
Oh, splendid, another “interactive” widget to dazzle us while we scroll past the actual science. Because what we really need in a medical article is a flashy calculator that pretends to solve the complexities of neuropharmacology with a few drop‑down menus. I’m sure choosing “young adult” or “elderly” will magically predict whether you’ll feel a twinge of anxiety after a single dose of cefaclor. And let’s not forget the delightful option to tick “low gut microbiome diversity”-as if we all have a detailed gut flora report sitting on our fridge. The whole thing feels more like a marketing gimmick than a genuine clinical aid, and it raises the question: who’s really benefiting from this slick interface? Probably not the patient.
Gena Thornton
Barbra makes a fair point about the presentation; however, the underlying intent of the calculator is to raise awareness of potential risk factors that clinicians might overlook. If we integrate such tools with electronic health records, they could prompt timely discussions between prescribers and patients, leading to more personalized care. The key is to use the calculator as a conversation starter rather than a definitive verdict.
Lynnett Winget
Adventure time for our gut! 🌈 When you pop a pill like cefaclor, it’s not just a boring chemical-it can throw a party in your microbiome, and sometimes the party gets a little too wild, messing with your mood. Think of it as a tiny orchestra where every instrument needs to be in tune; if a few violins (the good bacteria) miss a beat, you might feel a little off‑key. So, keeping a balanced diet rich in pre‑biotics and maybe a probiotic boost can help keep the symphony harmonious while you’re on antibiotics.
Amy Hamilton
Lynnett, your analogy beautifully captures the delicate interplay between antibiotics and the gut‑brain axis, reminding us that health is not merely the absence of disease but a dynamic equilibrium. Philosophically, we must view each prescription as an intervention in a complex system, demanding responsibility and foresight. Therefore, I assert that clinicians should adopt a precautionary principle, weighing the necessity of cefaclor against potential disruptions to this symbiotic harmony, and when possible, opting for narrow‑spectrum alternatives that preserve microbial diversity.
Lewis Lambert
Reading through all these perspectives feels like watching a medical drama unfold in real time-each character adding a layer of depth to the story of cefaclor and mental health. It’s powerful to see how a simple antibiotic can ripple through our bodies, minds, and even our philosophies. Let’s keep the dialogue alive and ensure patients get the safest care possible.
Write a comment
Your email address will not be published. Required fields are marked *