
Abiraterone Drug Interaction Checker
Check Drug Interactions
Enter a medication to check potential interactions with Abiraterone. The tool focuses on CYP3A4 interactions as described in clinical guidelines.
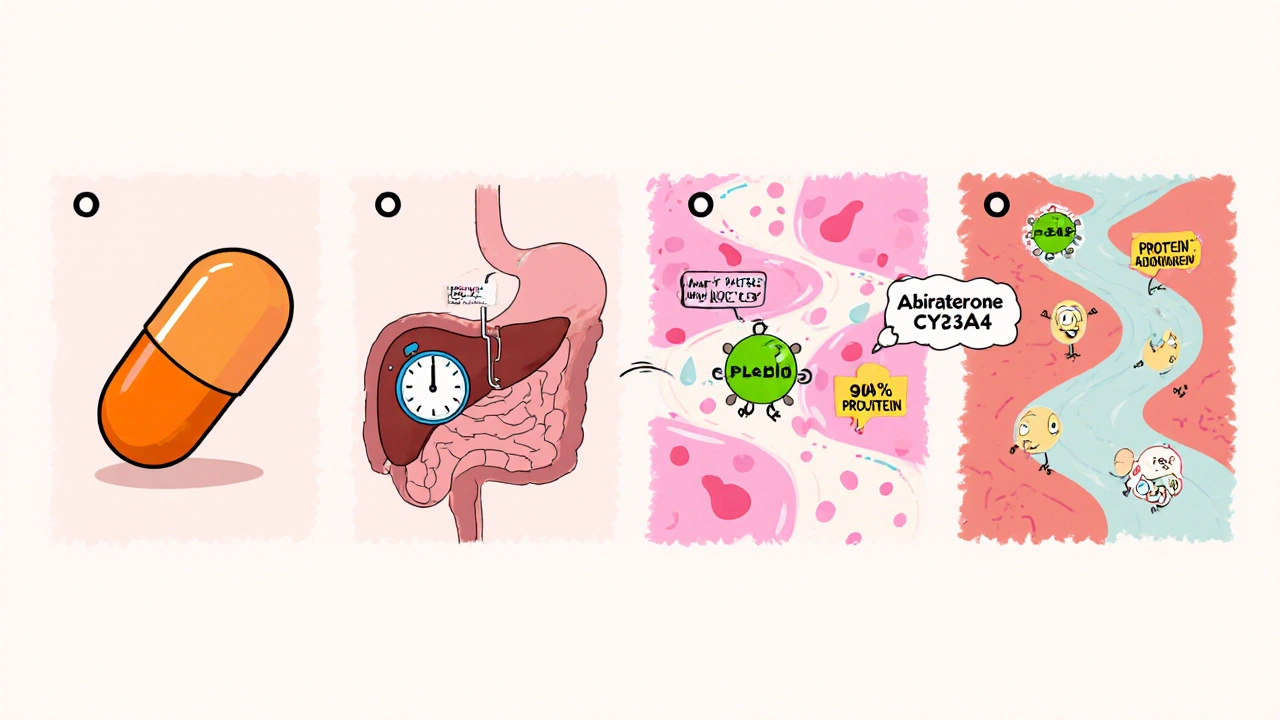
When doctors treat advanced prostate cancer, they often turn to a drug called Abiraterone is a steroidal inhibitor that blocks the enzyme CYP17A1, cutting down the body’s production of testosterone and other androgens. Understanding how this molecule moves through the body (pharmacokinetics) and what it does once it reaches its targets (pharmacodynamics) is key to using it safely and effectively.
Key Takeaways
- Abiraterone is absorbed slowly, reaches peak plasma levels in 2-3hours, and has a half‑life of about 12hours.
- It is primarily metabolized by CYP3A4 into the active metabolite Δ⁴‑abiraterone, which shares the same enzyme‑blocking activity.
- Because it lowers mineralocorticoid levels, patients need concurrent low‑dose steroids (e.g., prednisone) to avoid hypertension and hypokalemia.
- Food, especially high‑fat meals, can double the drug’s bioavailability; dosing on an empty stomach is recommended.
- Drug‑drug interactions are common with agents that induce or inhibit CYP3A4 or affect glucocorticoid metabolism.
Pharmacokinetic Profile
After oral administration, abiraterone is absorbed through the gastrointestinal tract. Its oral bioavailability is low (≈10%) because of extensive first‑pass metabolism, but a high‑fat meal can increase systemic exposure by up to 2‑fold. Peak plasma concentrations (Cmax) typically occur 2-3hours post‑dose.
The drug binds moderately to plasma proteins (≈96% albumin binding). Distribution volume is around 400L, indicating extensive tissue penetration, which is important for reaching androgen‑producing sites in the adrenal glands and tumor microenvironment.
Elimination follows a biphasic pattern: an initial rapid phase (t½ ≈ 4h) and a terminal phase (t½ ≈ 12h). About 85% of the dose is excreted in the feces as unchanged drug or metabolites; less than 3% appears in urine.
Metabolic Pathways
Abiraterone undergoes oxidation via CYP3A4 to form the active metabolite Δ⁴‑abiraterone. Both parent and metabolite are further reduced by 5α‑reductase and conjugated with glucuronic acid, facilitating biliary excretion.
Because CYP3A4 is a major route, inhibitors (e.g., ketoconazole, clarithromycin) can raise abiraterone levels and increase toxicity risk, while strong inducers (e.g., rifampin, carbamazepine) may lower exposure and reduce efficacy.
Pharmacodynamic Mechanism
The core action of abiraterone is to block CYP17A1, an enzyme that catalyzes both 17α‑hydroxylase and 17,20‑lyase steps in steroidogenesis. By inhibiting these steps, the synthesis of dehydroepiandrosterone (DHEA) and androstenedione drops dramatically, leading to reduced downstream testosterone and dihydrotestosterone (DHT) levels.
Lower androgen levels starve prostate cancer cells that rely on the androgen receptor (AR) signaling axis. However, the block also reduces cortisol synthesis, diverting precursors toward mineralocorticoid production. This explains why patients often develop hypertension, fluid retention, and hypokalemia unless they receive supplemental glucocorticoids.
Clinical Implications of PK/PD
- Dosing schedule: 1,000mg orally once daily on an empty stomach; prednisone 5mg twice daily to offset mineralocorticoid excess.
- Therapeutic monitoring: While routine plasma level checks are uncommon, clinicians watch for signs of adrenal insufficiency and adjust steroid dosing accordingly.
- Drug interactions: Avoid concurrent strong CYP3A4 inducers; consider dose adjustments if unavoidable.
- Renal or hepatic impairment: No dosage change is required for mild‑moderate liver disease; severe hepatic dysfunction (Child‑Pugh C) is a contraindication.
Comparison with Other Androgen‑Targeting Agents
| Attribute | Abiraterone | Enzalutamide |
|---|---|---|
| Mechanism | CYP17A1 inhibition (reduces androgen synthesis) | Androgen‑receptor antagonist (blocks AR signaling) |
| Oral Bioavailability | ~10% (food‑sensitive) | ~62% |
| Half‑life | ≈12h | ≈5.8d |
| FDA Approval Year | 2011 | 2012 |
| Common Side Effects | Hypertension, hypokalemia, liver‑function elevation | Fatigue, seizures, falls |
| Need for Steroid Co‑therapy | Yes (prednisone) | No |
Both drugs improve overall survival in metastatic castration‑resistant prostate cancer, but their PK/PD differences guide patient‑specific choices. Patients prone to cardiovascular issues may favor enzalutamide, while those with a history of seizures might do better on abiraterone.

Potential Pitfalls and How to Avoid Them
- Missing the empty‑stomach rule: Taking the pill with food can double exposure, leading to severe hypertension or liver toxicity. Educate patients to wait at least 1hour after eating.
- Ignoring drug interactions: Always review the medication list for CYP3A4 inducers or strong inhibitors. If a patient must start a potent inducer, consider switching to an alternative androgen‑targeting therapy.
- Under‑dosing steroids: Inadequate prednisone can precipitate adrenal crisis. Monitor blood pressure and potassium; increase steroid dose if needed.
- Overlooking liver function: Baseline ALT/AST should be checked, then every 4-6weeks. Significant elevation (>3× ULN) warrants dose interruption.
Future Directions in PK/PD Research
Recent phase‑II studies are testing a once‑daily, high‑fat‑meal‑compatible formulation of abiraterone that could simplify dosing. Additionally, pharmacogenomic profiling (e.g., CYP3A4*22 allele) may predict which patients will experience higher exposure and require dose adjustments.
Combination regimens with immune checkpoint inhibitors are also under investigation, aiming to exploit the immunomodulatory effects of reduced androgen levels.
Frequently Asked Questions
How long does it take for abiraterone to start working?
Patients usually notice a decline in PSA levels within 4-6weeks, but radiographic responses can take up to 3months.
Can abiraterone be taken with a high‑fat meal?
It can be, but the dose should be reduced by half and the patient must be monitored closely for hypertension and liver changes. The standard recommendation is to take it on an empty stomach.
Why is prednisone given alongside abiraterone?
Prednisone replaces the cortisol that drops when CYP17A1 is blocked, preventing excess mineralocorticoids that cause high blood pressure and low potassium.
What are the main drug interactions to watch for?
Strong CYP3A4 inhibitors (e.g., ketoconazole) increase abiraterone levels; strong inducers (e.g., rifampin) lower them. Antifungals, macrolide antibiotics, and certain antiepileptics fall into these categories.
Is dose adjustment needed for liver disease?
Mild to moderate hepatic impairment does not require a change, but severe liver failure (Child‑Pugh C) is a contraindication. Regular liver‑function tests are essential.



There are 12 Comments
Lin Zhao
The PK details you highlighted, especially the impact of high‑fat meals on bioavailability, are crucial for patient counseling 😊. Clinicians should remind patients to fast for at least an hour before dosing to avoid unintended spikes in plasma levels.
Additionally, monitoring electrolytes when prednisone is co‑prescribed can preempt hypertension or hypokalemia.
Albert Gesierich
While the overview is extensive, the statement that “abiraterone has a half‑life of about 12 hours” overlooks the biphasic elimination; the terminal phase actually extends to roughly 12 hours after an initial 4‑hour phase.
Moreover, the claim that “≤3 % appears in urine” should be qualified as “less than 3 % of the administered dose is excreted renally”. Accuracy matters when clinicians base dosing decisions on these parameters.
Suraj Midya
i get ur point but focus on the drug, not politics – the med facts matter more than any national agenda.
ashish ghone
When discussing abiraterone with patients, it helps to start by framing the therapy as a partnership against prostate cancer.
Explain that the drug works by blocking CYP17A1, which effectively starves the tumor of the testosterone it needs to grow.
Emphasize that because the enzyme also participates in cortisol synthesis, a low‑dose steroid such as prednisone is required to keep the hormonal balance in check.
Patients often worry about side effects, so reassure them that hypertension and low potassium are manageable with regular blood pressure checks and electrolyte monitoring.
Highlight the importance of taking the capsule on an empty stomach; a high‑fat meal can double exposure and increase the risk of those same side effects.
If a patient must eat, advise a dose reduction by half and close clinical follow‑up, as the literature suggests.
Regular liver function tests every 4‑6 weeks are advisable because hepatotoxicity, though uncommon, can appear silently.
In case of liver enzyme elevations beyond three times the upper limit, pause the medication and reassess.
For those on concurrent medications, review the list for strong CYP3A4 inducers like rifampin or carbamazepine, as they can markedly lower abiraterone levels.
Similarly, warn about potent inhibitors such as ketoconazole, which may raise exposure and precipitate toxicity.
Encourage patients to keep a simple medication diary, noting any new drugs, supplements, or over‑the‑counter products.
Educate them that even herbal remedies like St. John’s wort act as CYP3A4 inducers and should be avoided.
Remind them that adrenal suppression can present as fatigue or dizziness, signs that merit prompt medical attention.
For those with pre‑existing cardiovascular issues, a careful risk‑benefit discussion is essential before initiating therapy.
Lastly, discuss the upcoming research on meal‑compatible formulations, which may soon simplify the dosing regimen.
Staying informed empowers patients to adhere to treatment and report adverse events early, ultimately improving outcomes 😊.
steph carr
Thanks for laying out such a comprehensive patient‑education plan; I especially appreciate the suggestion to keep a medication diary, which can be a simple yet powerful tool.
Vera Barnwell
It never ceases to amaze me how the pharma giants push abiraterone as a miracle cure while quietly hiding the long‑term metabolic chaos it can unleash.
The half‑life data are presented as clean numbers, yet real‑world patients experience unpredictable spikes that mirror secretive dosing protocols.
One must wonder why the label emphasizes prednisone co‑therapy but glosses over the fact that chronic steroid use can itself trigger a cascade of immune suppression.
The hidden cost, however, lies in the subtle rise of cardiovascular disease that emerges months after the treatment begins.
Industry‑funded trials seldom report these outcomes in detail, opting instead for glossy survival curves.
There are whispers in the oncology community about undisclosed adverse events being filed away in confidential registries.
Even the FDA warnings mention only mild liver enzyme elevations, ignoring the cases where patients develop severe cholestasis.
I have heard of patients who, after years on abiraterone, develop irreversible adrenal insufficiency, a fate that is rarely discussed.
The interplay with CYP3A4 inducers and inhibitors is another layer of manipulation, allowing doctors to control drug levels without transparent guidelines.
When a high‑fat meal can double exposure, why isn’t this knowledge firmly embedded in patient handouts?
All these silences point to an orchestrated effort to keep the public in the dark while the profits keep climbing.
Stay vigilant, question every recommendation, and demand full disclosure of all side‑effect data.
David Ross
Indeed, the points you raise are compelling; the lack of full transparency does raise concerns, and clinicians should push for more exhaustive data releases, especially regarding cardiac and hepatic outcomes.
Henry Seaton
Drug interactions with CYP3A4 are the real deal, you must avoid strong inducers.
Baby Thingie
Correct, strong inducers like rifampin negate abiraterone’s efficacy. 😊
Abby Elizabeth
i cant believe how glossed over the side effects are, it's like they think we don't see the drama behind every lab spike and blood pressure surge, totally unfair!
Mark Haycox
the data actually show that only a minority of patients develop severe hypertension, the risk is quantifiable and manageable if monitored properly, so the panic is unwarranted.
Michael Taylor
I wholeheartedly agree with the emphasis on patient education, because informed patients are more likely to adhere to therapy, and adherence correlates directly with better outcomes.
Taking abiraterone on an empty stomach is not just a recommendation, it is a cornerstone of safe dosing, preventing unnecessary spikes in plasma concentration.
Monitoring blood pressure, electrolytes, and liver enzymes on a regular schedule creates a safety net that catches early signs of toxicity.
When a high‑fat meal doubles exposure, a dose reduction, as you mentioned, should be swiftly implemented, and the patient should be instructed to report any new symptoms immediately.
The role of prednisone cannot be overstated, as it mitigates mineralocorticoid excess, yet its dosing must be balanced to avoid its own side effects.
Drug‑drug interactions, especially with CYP3A4 modulators, demand a thorough medication review at each visit, because even over‑the‑counter supplements can tip the balance.
For patients with pre‑existing cardiovascular issues, a multidisciplinary approach involving cardiology can tailor the treatment plan to minimize risks.
Emerging formulations that are meal‑compatible hold promise, and participating in clinical trials may offer patients access to these advances.
Finally, encouraging patients to maintain a simple diary of doses, meals, and symptoms empowers them to become active partners in their care.
Healthcare providers should also stay updated with the latest guidelines, as recommendations evolve with new evidence.
In summary, a proactive, collaborative strategy ensures that the benefits of abiraterone outweigh its potential drawbacks, leading to improved quality of life.
Let’s keep the conversation going, sharing experiences and best practices, because together we can navigate the complexities of cancer therapy.
Write a comment
Your email address will not be published. Required fields are marked *